See also: Research
| Test Type | Result | Test Standard |
| Wind Driven Rain | Passed – 0.7oz (78% lower than the federal specification requirement) | ASTM D6904 |
| Vapour Permeability | 84 Perms | ASTM E96 (Method B) |
| Air Permeance | 0.002 CFM/ft2 | ASTM E2178 |
| Sorption Isotherm | 1.36% (90% Relative Humidity) | ASTM C1498 |
| Combustibility | Non-Combustible – NFPA 285 Exempt | ASTM E136 |
| Impact Resistance | 140 Pounds | ASTM D2794 |
| Accelerated Weathering | No color change, blistering, chalking, checking, cracking or other after 2000 hours | ASTM G154 |
| Thermal Conductivity | R-Value: 1.02 @ 3/8″ | ASTM C177 |
| Granulometry | 0.8 – 4.0 mm | ASTM C136 |
| Water Retention (on paste) | 94% (2% variance) | Meruc U4 |
| Dry Density (cured product) | 1450 g.1 +/100g/1 | EN 459-2 |
| VOC Content | 0 | ASTM D2369 |
| Capillarity | Between 1 and 2.5 g/dm2.min1/2 | EN 1015-18 |
| Modulus of Elasticity | 1.05 x 106 psi / 7239 MPa | ASTM C469 |
| Salt Fog Exposure | No Effect | ASTM B117 |
| Compressive Strength (28 days) | 1500 Psi at Full Cure | ASTM C109 |
| Flexural Strength (28 Days) | Between 2 and 2.5 N/mm2 | Meruc U4 |
| Solar Reflectance | Air Mass 1.5 – 0.8 / Thermal Emittance, 300K – 0.88 | ASTM E903 |
| Solar Reflectance Index (SRI) | Low, 5 W/m2K = 98.3
Medium, 12 W/m2K = 98.5 High 30 W/m2K = 98.8 |
ASTM E903 |
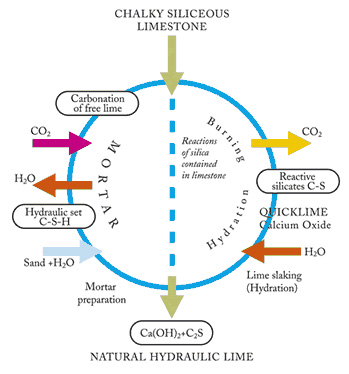
Hydraulicity is the property of a binder to harden in contact with water.
~ Hydraulicity is produced by burning a limestone containing silica, alumina, and iron oxides which above certain temperatures combine, totally or partially, with the Calcium Oxide. The resulting silicates, aluminates and ferrites give hydraulic properties to the product. Today as in the past, natural building limes are obtained by burning and slaking limestone and the more or less hydraulic character of the finished product is directly related to the percentage of calcium silicates, aluminates and ferrites formed during burning. The composition of the earth’s crust shows the predominance of silica and its presence is almost inevitable in all limestone deposits.
~ The building limes of the past, if the soluble (combined) silica content is analyzed, will almost certainly show some hydraulic property, even if very feeble. The analysis of historical finishes today rarely takes this factor into account and, as sometimes the amount of combined silica in a finish is minute, a number of findings will not identify the hydraulic component of the finish. For example: an amount of 4% of combined silica in a binder represents, in a typical finish with a 17.5% binder content, only about 0.7% of the total mass of the finish but still this finish will be feebly hydraulic.
Many characteristics of Generic Viagra production equipment affect the quality of purification.
~ The existence of pure calcium carbonate deposits is not common. High calcium limes are mainly exploited for industrial use (i.e. steel industry), where it is essential to have an almost pure material. Even in metamorphic type calcareous stone such as marble, silica is found. The little amount of silica required to combine with the CaO during the burning makes the production of hydraulic properties almost inevitable when the raw material is a calcareous stone. This finish will be almost certainly classified as non hydraulic by most analysts. If a “match” is required, this might be erroneously made by adopting a non hydraulic high calcium lime instead of a feebly hydraulic lime.
![]()
The Making of St. Astier Hydraulic Limes and Finishes
~ The quality of hydraulic limes derives from the mineralogical composition of the raw material and the manufacturer’s skill and production control.
~ The absence of sulfates in the St. Astier limestone and the low traces of alkali, such as potassium and sodium, cannot result in products which will flavor sulfate attack or alkali-silica reactions. The low amount of alumina will produce only very low levels of tricalcium aluminates, so important in avoiding sulfate attack. Chart 1 clearly shows the potential damaging components in binders which are responsible for many of the long term deterioration and failures in finishes.
The result of an efficient burning and controlled slaking is that St. Astier products have a high percentage of free lime residual very much above the minimum limits required by the standards for hydraulic limes.
A number of classifications/theories have been put forward. The main ones are listed below, together with their shortcomings:
1) Classification related to setting time.
It is based on the principle that limes with a setting time over 1 day are not hydraulic. The relevant tests are conducted on a lime paste and therefore cannot be acceptable as hydraulic limes are used in finishes (lime + aggregates). The setting time in finishes depends not only from the hydraulic properties of the lime, but also from the volumetric ratio of the mortar mix and other factors such as water content.
2) The cementation index.
It supposes that there is no unburned residue and that combined silica (SiO2) is present as C3S. Although this is correct in cement, it is not so in the case of hydraulic limes where C2S is the main hydraulic component and there is always an unburned residue. A high level of C3S would not allow hydraulic limes to be reworked as, for example, possible with St. Astier limes.
3) Classification based on color.
They were dismissed by their own authors.
4) Vicat classification.
In the early 19th century L. Vicat established that limestone containing silica, alumina, and iron oxides would produce hydraulic limes. He attributed the presence of these to “clay” impurities in the limestone and proceeded to classify in relation to the amount of “clay” content in a calcareous stone. He based his Hydraulicity Index on the following formula:
I = (SiO2total+Al2O3+Fe2O3) / (CaO total)
Vicat did not consider, however, that all the SiO2 is soluble (some of it is un-soluble quartz) and therefore available to combine with CaO. Furthermore, he supposes that all the CaCO3 in the stone is converted in CaO during the burning with no residue, which is also incorrect. Vicat formula is perfectly applicable to cement where the high burning temperature ensures that all components are combined in their near totality with the CaO, but cannot be today adopted for hydraulic limes. For example, using the Vicat Hydraulic Index, cement has an Index (I) of 0.42 with a compressive strength of approximately 55 N/mm2 @ 28 days and an NHL would have an Index of 0.37 with a compressive strength of 50 N/mm2.
The theory of soluble (combined) Silica.
~ This is by far the most reliable method of classifying hydraulicity. The principle is simple but indisputable: the silica in a calcareous limestone is combinable or inert. The appropriate burning process determines the quantity of silica that will combine. This explains how much uniform deposit, such as St. Astier quarry, it is possible to obtain different hydraulic characteristics from the same stone. Soluble silica combines with CaO (ratio of approx. 1:3) during burning at 1652°-1832°F (900°-1,000°C), forming CS (calcium silicates) which are responsible for hydraulicity.
~ The amount of available silica in the stone is the determining factor. Limestone containing less than 4% will not produce hydraulic limes. From 4% and above hydraulicity will be generated in direct proportion to the combined amount between available silica and CaO.
Soluble silica and ancient finishes analysis.
~ The soluble silica theory is of great value when studying ancient finishes to try and individuate their more or less hydraulic behavior. Once it is agreed that the soluble silica combines with the CaO to produce reactive calcium silicates, by finding the levels of soluble silica in ancient finishes, one can establish their degree of Hydraulicity and match them if so required. By using this method it will be surprising how many ancient finishes would show hydraulic properties. This is due to the fact that our forbears were making building lime with limestone rarely free of silica, alumina and iron oxides (minerals present in clay, hence the popular definition of “clay contamination”). As said previously, it would be enough for the soluble silica to be as low as 4% to generate hydraulic properties in the lime.
Consideration on pozzolanic additions to achieve setting in finishes not made with hydraulic limes.
~ Due to the properties of today’s Air Limes (putty or hydrated), the use of pozzolans is necessary in the majority of cases to allow the builder to get on with his work, but the attention to be paid to the water content in the mix, the variable setting properties, the granulometric and color requirements, result in unnecessary complication, higher costs and high potential risk of failure.
~ The use of pozzolans is not needed with Thermocromex. If the main reason for the pozzolanic material is to create a hydraulic effect then the use of the correct grade of natural hydraulic lime will achieve the same or better result in a safe and reliable manner.
~ The composition of the raw material, the experience of the manufacturer in the production process and the quality control procedures have made available to the user a range of Natural Hydraulic Limes suitable for all construction requirements.
![]()
Here are some reasons why Thermocromex is widely accepted and appreciated:
Purity – NO ADDITION of any kind is made to Thermocromex to enhance its performance.
No need for blending – Thermocromex permits the builder to select the most suitable product for the work at hand without having to add pozzolans, cement, plasticizers, water retainers, water proofers, etc.
Blending introduces considerable risks of errors, added costs and final short and long term results which are uncertain and therefore hazardous.
Compatibility – The availability of a range of pure binders with different performance characteristics ensures the compatibility of Thermocromex with existing finishes whatever their age.
Free Lime Content (available lime) – Responsible for workability and self healing in Thermocromex.
Economy – Generally binders are bought by weight, but mixed by volume, their bulk density therefore determines the volume used. The lower the bulk density the less will be the weight of product used when mixing by volume. The low bulk density of all Thermocromex that when comparing with cement, lime putties and some other hydraulic limes, with the same weight of material purchased one can obtain a sizably larger quantity of finish.
Versatility of use: Building, rendering and plastering finishes, grouts, injection, concrete, paints are all uses that can be achieved with Thermocromex.
|
Elasticity |
A factor in building without joints. Important in diminishing shrinkage cracking. |
|
Permeability |
Good vapor exchange qualities allow for condensation dispersion. No rot. Great benefits to the living environment. |
|
Resistance to salts |
The absence of any potentially damaging additions (i.e. gypsum or cement) make sulfate attack, alkali-silica reactions impossible. |
|
Suitable Compressive Strength |
Unlike cement or cementitious mixes (1:1:6 etc.) the compressive strength will be achieved gradually, allowing for movement. The availability of range will permit the making of finishes with the required strength without having to add or blend. |
|
Resistance to weather |
Early setting will provide quicker protection from adverse weather. |
|
Resistance to Bacteria and Vegetable growth |
The alkalinity of the finish does not favor their development. |
|
Insulation |
R-Value is 5 times greater than stucco. |
|
Color Uniformity |
The whiteness factor of Thermocromex always remains the same. |
|
LEED |
Thermocromex is eligible for 44 LEED points in 9 categories. |
|
CO2 Absorption |
Probably the most eco friendly contribution of using limes. Damaging CO2 is reabsorbed during the carbonation of the free lime. |
![]()
Hydraulicity and Properties – Chart 1
| % in content | ||||
|
Ordinary Portland Cement |
Thermocromex |
Potential damaging effect |
||
| Tricalcium Aluminate | C3A | 3 – 10+ | 0-1 | Reacts with Sulfates and water producing sulfate attack causing finish deterioration and eventual failure. Reacts with sea salts. Affects bricks/stones. |
| Tetracalcium Aluminoferite | C4AF | 8 – 10 | 0 | Reacts with Gypsum causing expansion |
| Sulfates | SO3 | 2 – 7 | 0.4 -0.6 | Contributes to sulfate attack |
| Alkalis | Na2O/K2O | 1 – 3 | trace | Reacts with the silicates in cement and sand producing gradual disintegration ALKALI-SILICA REACTION |
| Gypsum | CaSO4 | 2 – 9 | 0 | Subject to expansion, efflorescence. Deteriorates in contact with sea salt |
Note: in marine locations the air contains sea salt which reacts with C3A in OPC, causing serious damage to cementitious plasters.
Hydraulicity and Properties – Chart 2
![]()
Mineralogy of binders and the effects of free lime content and cement addition in lime finishes.
This document is meant to assist the user in the evaluation of the composition of various binders and finishes.
1. Premise
It is of the upmost importance that finishes are compatible, chemically and mechanically, with the existing substrates and with the building materials present.
The main damaging components are:
*Soluble salts (sulfates are the main ones) – Represented mainly by SO3, SO4, Ca SO4 (gypsum).
*Aluminates – Mainly tricalcium aluminate (C3A)
*The presence of sulfates and aluminates will cause, in relatively short period, a sulfate attack when these components are exposed to rain water.
*Alkalis (K and Na) – Their presence, even if generally in small percentages, can cause a reaction with the silica content of the sands (Alkali-silica reaction) with smaller effects of a sulfate attack.
*Organic content – It must be below 1% of the binder content. Organic matter is subject to deterioration in time and can also promote biological growth.
Mainly because of the above, it is by far preferable to adopt pure lime finishes.
Pure limes are of two categories:
A – calcium/magnesium (dolomitic) limes (air limes) – Their hardening is the result of their re-absorption of CO2 from the air (carbonation), therefore they are classified as air limes under the current EN/BS 459.1. They are produced from the burning and slaking of calcareous*/dolomitic stone with little or no content of minerals such as silica, alumina, and iron oxide.
*a stone is defined as calcareous when it contains more than 50% calcium carbonate (CaCO3)
~ The hardening is slow and can be further impeded by the presence of moisture (dampness or rain) which will not allow the surface of the finish to absorb CO2 due to the formation of a “film” on the surface itself. To obtain a set, in these products, addition of cement and possibly pozzolanic materials are made. These materials such as furnace slag, fly ashes, brick dust, residuals from ceramic production and so on are all grouped under the generic term of Pozzolans and their adoption is nearly justified by the use of volcanic ash that Romans adopted. The ash was from Vesuvius and was called Pozzolan because it was quarried in Pozzuoli, near Naples.
B – Hydraulic limes – So called because they set in contact with water (secondary hardening also takes place in contact with air due to the presence of air lime in their composition). They are produced from the burning and slaking of calcareous stone containing silica, aluminates, and sometimes ferrite.* The resulting calcium silicates, aluminates, and ferrites constitute the hydraulic component.
*As little as 2% silica content in the stone is sufficient to produce hydraulic characteristics. Silica represents 60% of the earth’s crust and therefore its presence in stones and clays is quite normal. Alumina and iron oxide content is normally much less than silica.
~ The EN/BS 459.1 does not allow any additions to the pure and natural hydraulic limes (NHL). If the cement or pozzolans are added in a quantity of up to 20%, the suffix “Z” has to be added and printed on the bag (NHL-Z). If the addition is over 20% or if various products such as cement, air lime, filler etc. are blended to produce a binder that sets in contact with water (hydraulic), the product is called HL.
![]()
2. Mineralogical difference between natural hydraulic lime (NHL), high calcium lime (CL/DL) and grey cement.
The main components of NHL’s are:
Hydraulic Elements
- Predominantly C2S (Belite)
- Some C3S or Alite (derived from possible “high spots” during burning)
- Calcium aluminates (Ca. 2%)
- Ferrites (<1%)
- Uncombined reactive silica. Portion of SiO2 which has become reactive (amorphous) due to exposure to heat, but has not combined with the CaO to form calcium silicates (CS). See Annex A.
Non Hydraulic Elements
- Available lime (free lime). Ca(OH)â‚‚ or Portlandite
Other Elements
- Alkalis (Na,K) between 0.04% and 0.07% for Na2O and 0.10% to 0.25% for K2O.
- Sulfates (SO4) derived from the natural clay content in the stone or from the type of fuel or coal used in the furnaces.
- As for Alkalis, the lower the figure the better.
- Unburned residuals. This fraction is inert.
The main components of high calcium limes are:
- Calcium hydroxide – Ca(OH)2 between 70 and 95%
- CaCO3 – limestone residual
The main components of grey cement are:
- Predominantly C3S
- Some C4S
- Aluminates 10-15%
- Gypsum/CaSO4: 5-10%
- Alkalis (NaK) > 2%
- Fillers
The main differences between NHL’s and cement are:
A. Chemical:
A.1 – Ratio between C2S (Belite) and C3S (Alite) and correspondent differences in compounds formed during hydration.
~ The hydration of both compounds produces the group CSH or Calcium Silicate Hydrates resulting in similar mechanical performances, but C3S will produce 3.2 times more than Portlandite [Ca(OH)2] than C2S. See full chemical reaction path on Annex B.
~ This Portlandite will be produced as soon as water is added to the material and in time, it will crystallize in the pores of the finish leading to reduced vapor permeability more commonly described as breathability.
~ The crystallization of Portlandite will also alter the elasticity moduli of the finish and stiffens finish creating a high risk of long term crack formation.
~ The presence in NHL’s of amorphous/reactive silica not combined with the CaO is beneficial as it fixes Portlandite, limiting the risk of efflorescence caused by Ca(OH)2 (Portlandite) leaching.
A.2 – Effects of high aluminate content (C3A) and sulfate (gypsum) addition to cement.
~ Aluminates produce delayed ettringite* by reaction with sulfates which are contained in the binder or in the building fabric and rain water. Sulfates addition in cement is necessary to slow the otherwise near instantaneous set. Otherwise known as “sulfate attack”, this most common problem causes the deterioration of the finish. The expansion force of the ettringite salt is much higher than the cohesion force of the finish (70-240MPa versus 4-6MPa), causing crack, joint delamination with consequent water ingress etc… For the chemical reaction path, see Annex C.
*Ettringite: calcium hydrate of aluminate tri-sulfate, also called Candlot salt.
A.3 – Effects of presence of Alkalis
~ Although the sodium oxide (Na2O) and potassium oxide (K2O) or alkali content in cement seems little (>2%), their presence can trigger the “alkali-silica” reaction, attacking the sand component in a finish. Alkalis are added to cement as flux materials to reduce the fusion temperature. They stay in the cement and can dissolve the silica, causing deterioration of the sand used in the finish.
A.4 – CO2 re-absorption
~ Cement does not re-absorb any CO2 emitted during production. As a consequence, blended cement/CL binders and finishes have a lower CO2 re-absorption value than NHL binders and finishes.
Table 1 – Summary of CO2 emission
|
Product |
CO2 Measured in Kg per Ton of Product |
||
|
Total CO2 emission |
CO2 re-absorbed |
Total CO2 |
|
| Cement | 819 | NIL | 819 |
| Hydrated Lime (CL) | 872 | 535 | 337 |
| Thermocromex | 606 | 270 | 336 |
For the complete table see Annex D.
The CO2 emission (CO2 not re-absorbed) of high calcium limes and NHL’s is quite similar. This is due to the higher total emission in the production of CL’s.
![]()
B. Physical
B.1 – Rheology, hardening kinetic
~ The great advantage of an alitic binder (cement based binder containing C3S as its main component) is its fast setting and hardening compared to belitic binders (NHL binder containing mainly C2S or belite). However, the consequence of achieving high mechanical performance rapidly is higher stress on the support and higher risk of shear. A belitic binder strength gain is more gradual as shown in the following table.
Table 2 – Comparison of hardening of Belite and Alite (hardening of pure components expressed in Kg/cm²)
Table 2 – Comparison of hardening of Belite and Alite
(hardening of pure components expressed in Kg/cm2)
|
Hardening Comparison |
7 Days |
28 Days |
180 Days |
365 Days |
|
Alite C3S |
322 | 466 | 512 | 584 |
|
Belite b-C2S |
24 | 42 | 194 | 325 |
From the table above we see that the alite long term strength is higher than belite. However, alitic binders (cement based) are much less elastic and breathable that belitic binders (NHL’s) as shown in the paragraphs that follow.
B.2 – Elasticity moduli
~ The crystallization of Portlandite produced in the hydration of C3S (as seen in A1) and by adding CL to cement (1:1:6 mixes etc…) has the effect of stiffening the finish. A hard finish will not accept movements. Control joints are necessary not only in pure cement finishes, but in all blended finishes containing cement.
B.3 – Permeability to vapor (breathability)
~ Permeability is extremely important as it diminishes condensation and the consequences of dampness. The void structure of cement finishes, especially when badly graded sands are used, is poor. The finish is quite dense. The capability of a finish to allow air movement in a structure is quite reduced in cement based finishes. Expressed in grams of air x m² x hour, concrete will have a value of 0.15 whilst NHL 5 and NHL 3.5 finishes made with ISO 679 standard at 1:2 ratio reach 0.55 and 0.64 respectively (up to 4 times better).
B.4 – Capillarity and water absorption
~ Cement finishes have a lower capillarity and water absorption than NHL and CL finishes, but their density and correspondent low permeability will promote moisture accumulation within the structure. High capillarity and water absorption are partly compensated by evaporation when a finish has a good void structure and high permeability to vapor. This is however not the case in cement finishes with their typical poor void structure.
B.5 – Bonding strength
~ The higher the bond strength of cement finishes does not necessarily mean higher durability. Dense finishes can (and do) delaminate if, for any reason such as shrinkage or movement cracks, there is moisture penetration and frost heave. The bonding strength of NHL’s is very adequate and the higher permeability and elasticity will result in better durability. The high bonding strength of cement finishes can also cause damage to the building elements when attempts are made to remove these finishes or when they come off by delamination. The face of the bricks, for example, remains attached to the cement finish. If a brick looses the face, its water absorption becomes much greater and the resulting high suction can cause problems even if the cement finish is replaced by a lime finish. Masonry elements built or plastered with cement finishes are rarely recyclable (in the sense of being reusable) as breakages occur when trying to take the cement finish off.
3. Major characteristics and properties of NHL – NHL/CL and OPC/CL finishes related to free lime content (available lime).
~ FREE LIME CONTENT – %Ca(OH)2 – directly influences a number of properties of finishes such as: plasticity, fineness, water demand, micro fissures, compressive strength, suction, water absorption, capillarity, vapor permeability, carbonation and lime leaching, and bonding strength.
Table 3
|
St. Astier lime Finishes |
CL Finishes |
CL + cement blends |
|
Thermocromex 8.2% |
23.1 to 30% |
1 : 1 : 6 16.5% |
~ Water lubricates the binder particles, governing plasticity. Workable site finishes have an average consistency with a flow between 180mm and 210mm (vibrating table – EN/BS 1015-3 test is conducted at 190mm). Flow values below or above these figures will produce, respectively, unworkable or unusable fluid finishes.
~ CL binders are finer than NHL’s (average 10 microns versus 50 microns) so more water is required with CL binders to achieve the necessary plasticity as it has to cover a greater number of particles. The greater the amount of water the greater the shrinkage and its related higher to suction and loss of strength. The simple explanation is that water evaporates, creating voids, resulting in shrinkage stress within the finish. When the stress is superior to the cohesion strength, micro cracks occur. At the surface these would barely visible, but they are contained mainly within the finish matrix. CL binders demand more water and its evaporation will increase the shrinkage stress within the finish resulting in weak finishes, requiring protection against the frost for a long period (often up to a 1 year, depending on the thickness of the finish and the exposure zone) as the finishes rarely reach a compressive strength above 2 N.mm².
~ The induced porosity described also produces higher suction due to the capillarity system of the micro cracks, allowing rain water penetration which not only shows carbonation of the free lime, but also frost/thaw heave in cold climates. It is the capillary structure that promotes water permeability (absorption) as the micro cracks on their won represent only a small part of the poor structure of the finish.
~ The effect of high free lime content on capillarity and water absorption is quite clear in Table 4 where, for example, an OPC/CL blend has higher capillarity and shrinkage when the free lime content is increased (1:2:9 versus 1:1:6) and lower permeability when cement is present due to Portlandite crystallization within the void structure.
Table 4 -Capillarity/Shrinkage/Vapor Permeability Binder: sand ratio 1:3
|
Capillarity/Shrinkage/Vapor Permeability |
St. Astier Finishes |
CL + cement blends OPC: CL: sand |
|
| Capillarity: g.min (@ complete carbonation |
Thermocromex |
1:1:6 1:2:9 |
|
| Shrinkage mm.m 28 days |
0.25 |
0.63 0.42 |
|
| Vapor permeability g.air.m2.h @ complete carbonation |
0.72 |
0.23 0.25 |
~ Pure CL finishes have a higher vapor permeability than NHL and blended finishes which would partly compensate for higher water absorption, but they are more susceptible to adverse climatic conditions for a much longer period of time. Their hardening time is prolonged and they need several interventions to prevent high shrinkage.
~ Carbonation of free lime is directly related to the finish exposure to air. Simply explained, limestone (CaCO3) during burning releases CO2 and becomes CaO. To harden*, it needs to re-absorb CO2 from the air, returning therefore releases CaCO3. This process is called carbonation. Factors impeding carbonation are surface damp (formation of a surface patina impeding contact with air), surface water (rain), non breathable surface coatings and finishes. The presence of un-carbonated free lime causes lime leaching if water is also present because of un-carbonated free lime is partly soluble in water at a ratio of 1.6g per liter (less in presence of other salts).
*Hardening is not to be confused with setting. It takes much longer and is related to carbonation of free lime and, in hydraulic binders, full development of belitic and alitic reactions.
The bonding strength of finishes, so important in rendering and plastering work, is also related to the free lime content in the binder.
~ The average bonding strength of pure CL 90 finish will be much lower than the 50/50 NHL-CL finishes. It might reach the minimum requirement of 0.3 N.mm² at full carbonation, but this would expose the work to potential weather damage for too long a period if one considers that the carbonation of pure CL is about 5-10mm per year.
![]()
Conclusion
Although the presence of free lime helps the workability of a finish, the higher the amount of free lime, the higher the water demand, capillarity, water absorption shrinkage, setting and hardening time. The bonding strength and frost resistance are lower.
The higher the free lime content the more careful should be the consideration given to the application season and protection of these finishes, especially in high rain exposure zone and in cold climates.
The addition of pozzolanic materials will enhance the setting properties of finishes with high free lime content, but not much is known about the long term complete effect of pozzolanic additions on the other properties and the overall durability of these finishes.
If, to enhance the setting, cement is added in the case of a number of finishes widely used, there is a detrimental effect due to the composition of cement and the other properties of cementitious finishes as shown earlier in this document.
It is essential to achieve compatibility of new material with the existing ones. In many situations weak finishes or finishes with high free lime content are required. When looking at the possibility of using these finishes the following considerations should be made:
- Site exposure
- Application season
- Protection and curing
- Condition of the building structure (good or friable etc…) and its detailing
- Walls dampness and causes
- Presence of salts
- Choice of aggregates
- Void structure, vapor permeability and capillarity requirements f the new finish/s.
- Thickness and depth of the joints (carbonation time of building – pointing finishes) or size and structure of voids.
- Thickness of the finishes (thin finishes with free high lime content will carbonate quicker than thick finishes).
- Workability and ability of the contractor to comply with the specifications issued
The responsibility of the specifier is to understand the intervention needed and choose the most suitable finish/s for the work to be done. The responsibility of the supplier is to provide correct and in depth information to assist the specifier in making the correct choice. The responsibility of the contractor is to provide the necessary skills and follow the correct working practice.
Potential failures are possible if the composition of the binder/s to be used is not known or understood, if additions (cement, pozzolans, and additives) are made without knowing their long term effects, if superficial knowledge is mistaken for professional knowledge and if price considerations prevail on technical ones.
Ugo Spano/Laurent Tedeschi
September 2006
![]()
Summary of formation and effects of reactive
All natural limes are produced in industries by calcinations of calcareous rocks at temperature ranging from 900°C to 1300°C. Below these temperatures, carbonation (1) yield is not sufficient and at high temperatures, lime crystals will combine back and the resulting quick limes will be hard to slake. For natural limes with hydraulic properties, there is formation of over burned CaO which explains how slaking NHL lime is slower than slaking CL’s. The slaking reaction of NHL passes through a maturing phase as described by M LE CHATELIER. In this phase all CXS are preserved.
(1) Ca CO3 + (Energy) → CaO + CO2
If calcium oxide or quick lime is in contact with silica, combination will result. This reaction is exothermic.
(2) 2CaO + Si 02 → (CaO)2 Si O2 + energy
In other words, this reaction gives energy in opposition to de-carbonation reaction (1) which is endothermic (takes energy from the system to happen). It is necessary to heat limestone to produce lime, but as soon as this lime is produced, it combines exothermically which hydraulic elements present.
Limes are generally produced in vertical kilns so material must stay solid form through the whole length of the kiln in contrast to cement production where horizontal kilns are used and material is transformed through a liquid state (fusion).
Silica is present in limestone as small inclusion particles (>1 mm) and has only a superficial contact with calcium oxide. Combination reactions are only produced on the surface of silica particles. The internal silica particles do not combine, but have been heated and the surface combination reaction is exothermic (2).
Original silica particles in the quartz crystalline state (sand) change to amorphous state (pozzolan, basalt, pumice) by the effect of heat. When these are crushed through grinding, amorphous silica becomes available. This amorphous silica is called “pozzolanic”. A very basic (Ph ˜ 12.5) mixture that brings amorphous silica in solution, as indicated by Boyton, is obtained when lime is mixed with water to prepare a finish.
(3) ΞSi – O – SiΞ + 3OH- → [SiO(OH)3]- …
It precipitates with free lime to produce:
(4) Y [SiO(OH)3]- + X Ca + + (Z-X-Y) H2O + 2(X-Y) OH- → CxSyHZ
The “types” of CHS (x, y and z values) obtained after reaction are related to the concentration of the present species, but the crystals do not correspond to CSH obtained by belite (C2S) crystallization. Theoretically these “types” can be identified, but in practice no exhaustive studies have been conducted. This pozzolanic gain is active or useful since the increase of mechanical performance can only be efficient if there is some hydraulicity in the system which is the case in natural hydraulic limes. For calcium limes, this hydraulicity is introduced by addition of cement or from endogenous activators in crushed pozzolans [Na2O, K2O (alkalis) with SiO2 in pozzolans].
In this table, a mixture of pozzolan is represented with no sand since alkalis would dissolve the sand. To obtain a measurable performance increase, CxSyHz must have seeds of CSH to grow; this is the crystalline germination step.
In conclusion: there will be no pozzolanic activity if there was no initial hydraulicity. Addition of amorphous silica to calcium lime will not provide measurable mechanical performance increases whereas additions to NHL2 can result in a very high compressive strength increase.
In an NHL finish, the influence of amorphous silica not initially combined to form calcium silicates, but made available through the crushing of some oversized slaked hydraulic lime granules is positive since it fixes Portlandite [Ca(OH)2] formed in the hydration of C2S and limits the risk of surface efflorescence caused by leaching of Ca(OH)2.
![]()
Difference in C2S v C3S hydration and Portlandite formation
During finish setting, CxS (C2S and C3S) will hydrate to produce « C-S-H » compounds according to the following equations (1) and (2)
Alite hydration (C3S) :
(1) 2C3S + 10.6 H → C3.4-S2-H8 + 2.6 CH
2(CaO)3 SiO2 + 10.6 H2O → (CaO)3.41 (SiO2)2, (H2O)8 + 2.6Ca(OH)2
MC3S = 228 g.mol-1 MCH = 74 g.mol-1
1 % in mass of C3S will produce 74*2.6/228*2 = 0.42% mass
Belite Hydration (C2S) :
(2) 2C2S + 8.6 H C3.4-S2-H8 + 0.6 CH
2 (CaO)2 SiO2 + 8. 6 H2O (CaO)3.41 (SiO2)2, (H2O)8 + 0.6 Ca(OH)2
MC2S = 172 g.mol-1 MCH = 74 g.mol-1
1 % in mass of C2S will produce 74*0.6/172*2 = 0.13% mass
Comparison of equation (1) and (2) leads to the following observations:
C3S and C2S will hydrate to form the same CSH resulting in similar mechanical performances but C3S will produce 3,2 times more Portlandite than C2S, increasing the risk of lime leaching.
![]()
Aluminates and sulfates: sulfate attack.
Aluminates can produce “delayed” ettringite by reaction with sulfates that are contained in ancient masonry work (gypsum roughcast, gypsum plasters on walls, capillary effect from sulfate water) or coming from urban pollution. Ettringite is a calcium hydrate of aluminate tri-sulfate with the following formulation:
C6AS3M32 [(CaO)6AI2 O3(SO3)3(H20)32] also called Candlot salt
Sulfates are added to cement to control its set time since the aluminates contained in cement have a very rapid set (measured in minutes) which causes a false set.
These sulfates are added in the form of gypsum that is “captured” by the aluminates at the beginning of hydration to form “premature” ettringite. These reactions are harmless towards final quality of the finish since its initial set has not yet started.
The following reactions are taking place (1)-(2) and (3)
(1) C3A + 3CSH2 + 26H → C6 HS3H32
Gypsum has been “consumed” by reaction (1) giving:
(2) 2C3A + C6ASH32 + 4H→ 3C4ASH12
Premature ettringite reacts to give:
(3) C3A + CH + 12 H → C4 A H13
These reactions are normal in a cement based component but if sulfates that are added to the finish after it has set, the following reactions occur:
(4) CH + SH → CSH
with C4ASH12 from equation (2) we obtain:
(5) C4 ASH12 + 2CSH + 1 6H → C6 AS3H32
|
M = 623 g.mol-1 d = 1990 kg.m-3 |
V mol = 1240 |
M = 1255 g.mol-1 d = 1750 kg.m-3 |
V mol = 2144 |
Crystal volume almost doubled 1240 → 2144
With C4 AH13 from equation (3) we get:
(6) C4AH13 + 2CSH + 14H → C6AS3 H32 + CH
|
M = 560 g.mol-1 d = 2050 kg.m-3 |
Vmol = 1148 |
Vmol = 2144 |
Again, crystal volume almost doubles 1148 → 2144
These expansion reactions take place when the finish has already set and is no more flexible.
The expansion caused by salt crystallization results in pressure that can be as high as 70 to 240 MPa according to some authors. This stress is considerably higher than the cohesion strength of a finish, which is in the order of 4 to 6 MPa.
![]()
CO2 EMISSION OF VARIOUS BINDERS
|
Material – Energy Requirements |
Fuel (coal) Kg/Ton |
Therms Per Ton |
Kilowatts/hour Per ton |
| Cement | 110 | 900 | 120 |
| Hydrated CL 90% | 84 | 650 | 50 |
| Thermocromex | 70 | 550 | 25 |
| Material |
CO2 per Kg/Tonne of Binder |
||||
|
Emission during burning |
Emission from |
Total emitted |
Re-absorbed |
Not Re-absorbed |
|
| Cement | 403 | 416 | 819 | NIL | 819 |
| Hydrated CL 90% | 308 | 564 | 872 | 535 | 337 |
| Thermocromex | 256 | 350 | 606 | 270 | 336 |
Research
St Astier Natural Hydraulic Limes (NHL)
Chemistry and mineralogy of the raw material
~ Manufacturing and finished products chemical and mineralogical data –
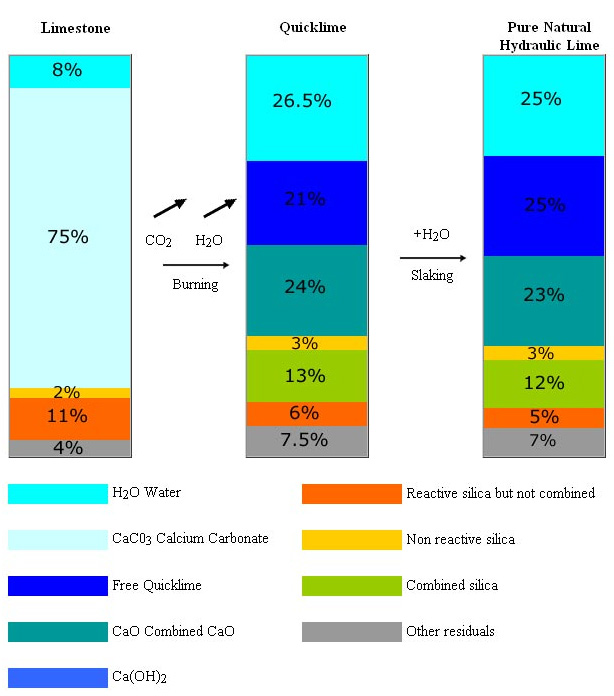
Thermocromex is produced from the burning and slaking of a pure chalky limestone with siliceous content. No additions are made. They strictly conform to the French Norm NFP 15.311 and the European Norm EN 459 classifying NHL.
~ The limestone in the St. Astier basin (approx. 40 Km2) derives from crustacean deposits (chalky limestone) infiltrated by silica but untouched by clay. Exploited for thousands of years, industrial production began in 1851. The quarries, owned by the same group from the industrial beginning, extend for 300,000 square meters (30 hectares). Tests conducted by the French government show a unique uniformity in the composition of the deposits (up to 100 m. depth).
Chemical and mineralogical analysis of the deposit:
|
Chemical Analysis |
Loss at ignition |
CaO |
SiO2 |
MgO |
Al2O3* |
Fe2O3* |
SO3* |
Na2O* |
K2O* |
Others* |
|
% |
40 |
44 |
13 |
0.6 |
1.1 |
0.32 |
0 |
0.04 |
0.1 |
0.84 |
- The absence of clay infiltration and the consequent minimal presence of Al2‚O3, sulfates and alkalis ensure the production of hydraulic limes based almost totally on the combination of Calcium Oxide and reactive silica.
|
Corresponding Mineralogical Composition |
H2O (moisture content) |
CaCO3 |
SiO2 (soluble) |
SiO2(insoluble) |
MgCO3 |
Others (derivatives from items marked * above) |
|
% |
8 |
75 |
11 |
2 |
1 |
3 |
|
Reactive/combinable |
Inert/un-combinable |
- The soluble silica, available to be combined with the CaO produced in the burning of the CaCO3 determines the hydraulicity of the finished products.
The production of different types of Natural Hydraulic Limes from the same raw material deposits proves that hydraulicity depends on the amount of silica combined and not on the total amount present. The theory that hydraulicity depends on the total amount of “clay (or silica)” in the raw material is fundamentally flawed.
The production method is essentially unchanged from the one used since ancient times: limestone burned and slaked. It is therefore correct to say that Thermocromex is amongst the very few traditionally produced limes. The scientific knowledge of the manufacturer and modern quality control has, however, the favorable effect of producing reliable materials with constant performance.
The burning process: Its methods and the energy used are the determining factors in the quantity of silica that combines with Calcium Oxide (CaO) to form Calcium Silicates (CS) which produces the hydraulic performance of the finished products. Burning takes place in vertical kilns at temperatures not above 1832oF (1,000oC). The fuel is anthracite coal, imported from Wales due to its purity, as it produces the least residuals.
Continuous checks are made to measure the efficiency of the burning (CO2 tests) which are essential to regulate the hydration that follows.
Hydration (slaking): The controlled hydration process is so precise that virtually no quick lime (<1%) will be present at the end. The efficiency of the slaking process is such that only a small percentage of the slaked material has to be milled to achieve the desired granulometry (0.09mm). As shown below, the amount of potentially damaging components produced is so minute that adverse reactions, leading to materials deterioration, are not possible.
* The presence of SO3, absent in the raw material, is induced by the coal used in burning. The small level of it, however, is harmless. Higher gypsum (CaSO4) levels due to additions as in the case of ordinary cement or some other hydraulic binders can cause damage.
C3S can occur due to “high spots” in the furnace and also due to eutectic reactions caused by the presence of alkalis which lowers the fusion point.
![]()
EU Norms (EN 459 Mandatory testing program for Natural Hydraulic Limes
|
POWDERS |
|||||
| TEST | NORM | EN459 Values | St Astier Values | Frequency EU Norms | St. Astier Frequency |
| Fineness | EN196-6 | residue by mass 0.09mm/0.2mm <15%/<5% |
5-7% | daily | Daily |
| Free water | EN459-2 | 3.5<2 | <2 | ||
| Soundness (Expansion) |
EN459-2 | <2 | <1 | once a day | several timesper day |
| Bulk density Kg/dm3 |
EN459-2 | NHL3.50.5-0.9 | 0.61/0.65 | monthly | each product batch |
|
FINISHES (prepared with standard EU sand/ISO 679 @ 1:1 vol. ratio |
|||||
| Penetration | EN459-2 | >20mm | >20mm | monthly | Weekly |
| <50mm | <50mm | ||||
| Setting time | EN459-2 | >1h/<15h | >3h/<10h | 2 per week | 2 per week |
| Air content | EN459-2 | <20 | <20 | monthly | monthly |
| Compressive | EN459-2 | 28 days | weekly | continuously | |
| Strength | N/mm2 | ||||
| NHL3.5 | 3.5 to 10 | 3.5 to 6 | |||
| SO3 | EN196-2 | ||||
| content % | 3* | 0.45/0.65 | monthly | continuously | |
| *up to 7% admissible if soundness is demonstrated at 28 days of water curing | |||||
| Available | EN459-2 | NHL53% | 15-20% | weekly | each product batch |
| Lime | minimum | NHL3.59% | 22-26% | each product batch |
|
| values | NHL215% | 50-55% | each product batch |
||
Other tests regularly performed by the St. Astier Research & Development Dept:
CO2 contents after each burning to regulate slaking, Tensile strength, Elasticity moduli, Adhesion, Workability (flow table), Whiteness.
![]()
Ecological Evaluation of Lime Binders using Natural Lime
CO2 re-absorption comparison for Thermocromex compared with similar Portland cement: Lime Finish Mixes
Carbon Dioxide deficit for 1 Cubic Meter of finish
|
Ecological Evaluation |
|||||
|
Amount of CO2 in Kilograms Emitted and Recovered per KG of Binder in the production and use of typical finishes. |
|||||
| Binder Constituents for Cement Finishes in a 1:1:5 ratio. (KGs per M3) | Binder Constituents for an equivalent St Astier Natural Hydraulic Lime Finish in a 2:5 ratio (KGs per M3) | ||||
| Sources of Carbon Dioxide | Portland Cement 240 KGs required | High Calcium Lime 83 KGs required | NHL 3.5 244 KGs required | ||
| Therms of energy used to produce 1 tonne of binder |
900 |
650 |
550 |
||
| A | CO2 generated from Fuel |
96.72 |
25.56 |
61.44 |
Base on coal requirements to produce 1 tonne of binder |
| B | CO2 Generated from Material (De- Carbonization) |
99.84 |
45.12 |
85.4 |
CO2 emission during manufacture |
| C | Re-Absorbed by Material |
Nil |
44.4 |
65.88 |
CO2 re-absorption during curing and first 2 years |
| CO2 Deficit – (A+B) – C KGs per M3 of material |
196.56 + 26.28 = 222.84 |
80.96 |
Value of CO2emission against re-absorption | ||
![]()
| PLASTER COUNCIL_______TECHNICAL BULLETIN #5 |
|---|
| Judging Installations |
| “As natural hand applied products stucco finishes cannot be expected to achieve perfection. This technical paper represents the view points of multiple stucco associations, applicators, consultants and technical experts, to offer guidance to industry professionals when evaluating plaster finishes.” |
| Distance: |
“Stand 10 feet from building to evaluate plaster texture, flatness, cracking and other appearance issues.”Panel Size:
“Any discrepancy may appear more significant on larger panels, relative to how they may appear on smaller panels. Thus plaster with large panels should be expected to show more imperfections.”Lighting:
“Keep in mind that light cast parallel to the wall or a small angle to the wall (including critical light) can make minor texture and flatness issues look significant.”Color:
“Smooth textures appear darker than rough surfaces. Light source affects perceived color as the same surface may take on a different appearance under daylight light sources. Reflected light from landscaping and other materials on a building can have a significant effect on the perceived color of the plaster.”
![]()
| PLASTER INSTALLATION CONSIDERATIONS |
|---|
| JUDGING THE FINISHED PRODUCT |
Applying plaster is and always has been a skill-intensive trade. The quality will depend largely on the knowledge and skill of the plasterers applying the plaster mix. Unlike a machine or factory-fabricated product, expecting machinelike perfection is impracticable and unreasonable. Applying plaster requires skilled tradesmen to apply the matrix in various weather conditions and often under unfavorable circumstances. The skill of the plasterer, weather conditions, time allowed to work, substrate quality and available materials all play a part in the quality of the finishes appearance.
STANDARDS:
A mock-up or sample wall should be approved by the owner before the project is started.
VIEWING:
Normal viewing distance is considered 10 to 15 feet from the surface to be judged. You should be able to observe some uniformity in the overall texture and color of the finished surface. Judging should be done under normal lighting conditions and cannot be limited to brief periods of time when the suns angle to the plastered surface creates a critical light condition. No plaster wall is perfectly flat. Strong light casting across the surface at just the right angle will make a good wall look bad, sometimes really bad.
EXAMPLE OF WALL AFFECTED BY CRITICAL LIGHT
 |
 |
| 8:30 AM – 8: 45 AM | Rest of Day |
Western Walls & Ceilings Constructors Association
Technical Services Information Bureau
1910 North Lime Street • Orange, CA 92865-4123 • Phone (714)221-5530 • Fax (714221-5535 • www.tsib.org)
![]()
| COMMENTS FROM VARIOUS PLASTER MANUFACTURERS: |
• “Uniformity of Finish: Despite the most meticulous application of any of these materials, it is important to understand that materials of this nature are a truly hand applied material and some planar irregularities of the wall surface should be expected. The intention of the information provided above is simply to provide applicators, designers and owners with a variety of methods which minimize the impact of the critical light phenomenon to assist in achieving the desired aesthetic result.”
• “Finish systems for the most part, applied by hand at the jobsite under varying environmental conditions, and not by a machine in a controlled atmosphere, so the resulting final surface will have some variations.”
• “Irregular appearance that results from aberrations in the wall plane can be moderated to some degree, by utilizing finishes with course or heavy textures. Since the thickness of a textured finish is determined by the size of the aggregate that is used, the courser textured finishes are thicker and therefore are more capable of filling depressions in the surface than a thinner, fine aggregate finish.”
• “The surface of large panels should be divided into smaller areas by means of rustications or reveals to minimize the perception of textural differences. As a general rule, a textured surface provides a better aesthetic finish than a smooth finish.”
![]()
* For further guidance, please contact Southwest Progressive Enterprises.
St. Astier Limestone High Performance Cladding – Thermocromex
~Based on St. Astier natural hydraulic lime, this product is visually pleasant, durable, water resistant and cost effective.
~ A one coat easy and economical application, ready mixed product based on Natural Hydraulic lime specially formulated to work on all substrates, including light weight cement blocks, clay blocks, bricks, and stone to achieve excellent waterproofing, low capillarity, high breathability, elasticity, and vibrant colored finishes. Thermocromex is available in a variety of colors. Our color chart is by no means exhaustive and can be supplied to a custom color with substantial savings in cost of painting and maintenance.
